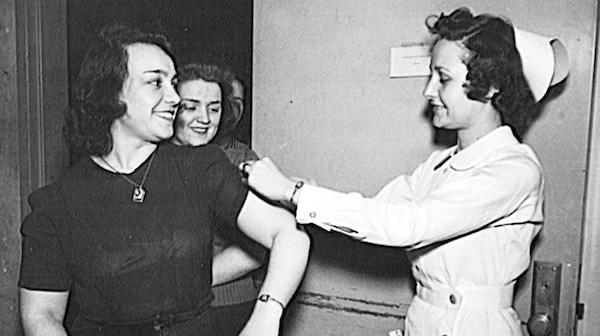
(FILES) In this file photo taken on December 12, 2002 (FILES) This file photo taken in January 1943 shows US Office of War Information (OWI) employees receiving free inoculations against smallpox, as well as diptheria and typhoid, in Washington, DC.
A member of the governmentís coronavirus taskforce says the Results from the UKís first human trial of a vaccine could be available by the middle of June.
According to Professor Sir John Bell, regius professor of medicine at Oxford University, researchers were waiting to see whether the volunteers catch the disease
Since testing began three weeks ago several hundred have been injected with a total of 1,100 participants expected to take part in the trial
Half the participants receive a potential vaccine while the other half, the control group, are to receive a commonly available meningitis vaccine.
Professor Bell said the Oxford University project, which is being lead by professor of vaccinology Sarah Gilbert, is currently going well and according to plan.
"Weíre now starting to wait for an advocacy signal to see whether people whoíve been vaccinated, donít get the disease so thatís the next step," he told BBC Radio 4ís Today programme.
He did, however, stipulate that there may not be sufficient active disease in the community for the participants to catch it naturally
"Weíre doing those calculations because we have quite good data now on how much disease there is around," he added.
He went on to explain that the researchers had rejected the idea of intentionally exposing people to the virus since the risk of death would be too high if the vaccine did not work.
The team, which has formed a partnership with AstraZeneca, expects to produce one million doses of the vaccine by September and to scale up manufacturing once it is approved by regulators.
Prof Bell said: "We also want to make sure that the rest of the world will be ready to make this vaccine at scale so that it gets to populations in developing countries, for example, where the need is very great.
"We really need a partner to do that and that partner has a big job in the UK because our manufacturing capacity in the UK for vaccines isnít where it needs to be, and so we are going to work together with AstraZeneca to improve that considerably."
SOURCE: PRESS TV
LINK: https://www.ansarpress.com/english/17535
TAGS:





























 Farkhunda Buried, Ghani Appoints Fact-Finding Team
Farkhunda Buried, Ghani Appoints Fact-Finding Team




